Detailed derivation of the energy balance on water:
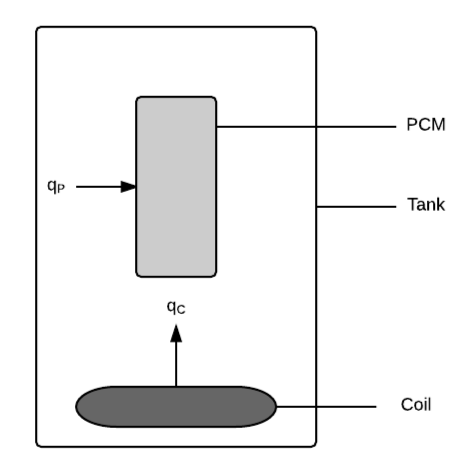
To find the rate of change of TW, we look at the energy balance on water. The volume being considered is the volume of water in the tank VW, which has mass mW and specific heat capacity, CW. Heat transfer occurs in the water from the heating coil as qC (GD:htFluxWaterFromCoil) and from the water into the PCM as qP (GD:htFluxPCMFromWater), over areas AC and AP, respectively. The thermal flux is constant over AC, since the temperature of the heating coil is assumed to not vary along its length (A:Temp-Heating-Coil-Constant-over-Length), and the thermal flux is constant over AP, since the temperature of the PCM is the same throughout its volume (A:Temp-PCM-Constant-Across-Volume) and the water is fully mixed (A:Constant-Water-Temp-Across-Tank). No heat transfer occurs to the outside of the tank, since it has been assumed to be perfectly insulated (A:Perfect-Insulation-Tank). Since the assumption is made that no internal heat is generated (A:No-Internal-Heat-Generation-By-Water-PCM), g = 0. Therefore, the equation for GD:rocTempSimp can be written as:
\[{m_{\text{W}}}\,{C_{\text{W}}}\,\frac{\,d{T_{\text{W}}}}{\,dt}={q_{\text{C}}}\,{A_{\text{C}}}-{q_{\text{P}}}\,{A_{\text{P}}}\]
Using GD:htFluxWaterFromCoil for qC and GD:htFluxPCMFromWater for qP, this can be written as:
\[{m_{\text{W}}}\,{C_{\text{W}}}\,\frac{\,d{T_{\text{W}}}}{\,dt}={h_{\text{C}}}\,{A_{\text{C}}}\,\left({T_{\text{C}}}-{T_{\text{W}}}\right)-{h_{\text{P}}}\,{A_{\text{P}}}\,\left({T_{\text{W}}}-{T_{\text{P}}}\right)\]
Dividing Equation (2) by mW CW, we obtain:
\[\frac{\,d{T_{\text{W}}}}{\,dt}=\frac{{h_{\text{C}}}\,{A_{\text{C}}}}{{m_{\text{W}}}\,{C_{\text{W}}}}\,\left({T_{\text{C}}}-{T_{\text{W}}}\right)-\frac{{h_{\text{P}}}\,{A_{\text{P}}}}{{m_{\text{W}}}\,{C_{\text{W}}}}\,\left({T_{\text{W}}}-{T_{\text{P}}}\right)\]
Factoring the negative sign out of the second term of the right-hand side (RHS) of Equation (3) and multiplying it by hC AC / hC AC yields:
\[\frac{\,d{T_{\text{W}}}}{\,dt}=\frac{{h_{\text{C}}}\,{A_{\text{C}}}}{{m_{\text{W}}}\,{C_{\text{W}}}}\,\left({T_{\text{C}}}-{T_{\text{W}}}\right)+\frac{{h_{\text{C}}}\,{A_{\text{C}}}}{{h_{\text{C}}}\,{A_{\text{C}}}}\,\frac{{h_{\text{P}}}\,{A_{\text{P}}}}{{m_{\text{W}}}\,{C_{\text{W}}}}\,\left({T_{\text{P}}}-{T_{\text{W}}}\right)\]
Rearranging this equation gives us:
\[\frac{\,d{T_{\text{W}}}}{\,dt}=\frac{{h_{\text{C}}}\,{A_{\text{C}}}}{{m_{\text{W}}}\,{C_{\text{W}}}}\,\left({T_{\text{C}}}-{T_{\text{W}}}\right)+\frac{{h_{\text{P}}}\,{A_{\text{P}}}}{{h_{\text{C}}}\,{A_{\text{C}}}}\,\frac{{h_{\text{C}}}\,{A_{\text{C}}}}{{m_{\text{W}}}\,{C_{\text{W}}}}\,\left({T_{\text{P}}}-{T_{\text{W}}}\right)\]
By substituting τW (from DD:balanceDecayRate) and η (from DD:balanceDecayTime), this can be written as:
\[\frac{\,d{T_{\text{W}}}}{\,dt}=\frac{1}{{τ_{\text{W}}}}\,\left({T_{\text{C}}}-{T_{\text{W}}}\right)+\frac{η}{{τ_{\text{W}}}}\,\left({T_{\text{P}}}-{T_{\text{W}}}\right)\]
Finally, factoring out \(\frac{1}{{τ_{\text{W}}}}\), we are left with the governing ODE for IM:eBalanceOnWtr:
\[\frac{\,d{T_{\text{W}}}}{\,dt}=\frac{1}{{τ_{\text{W}}}}\,\left({T_{\text{C}}}-{T_{\text{W}}}+η\,\left({T_{\text{P}}}-{T_{\text{W}}}\right)\right)\]